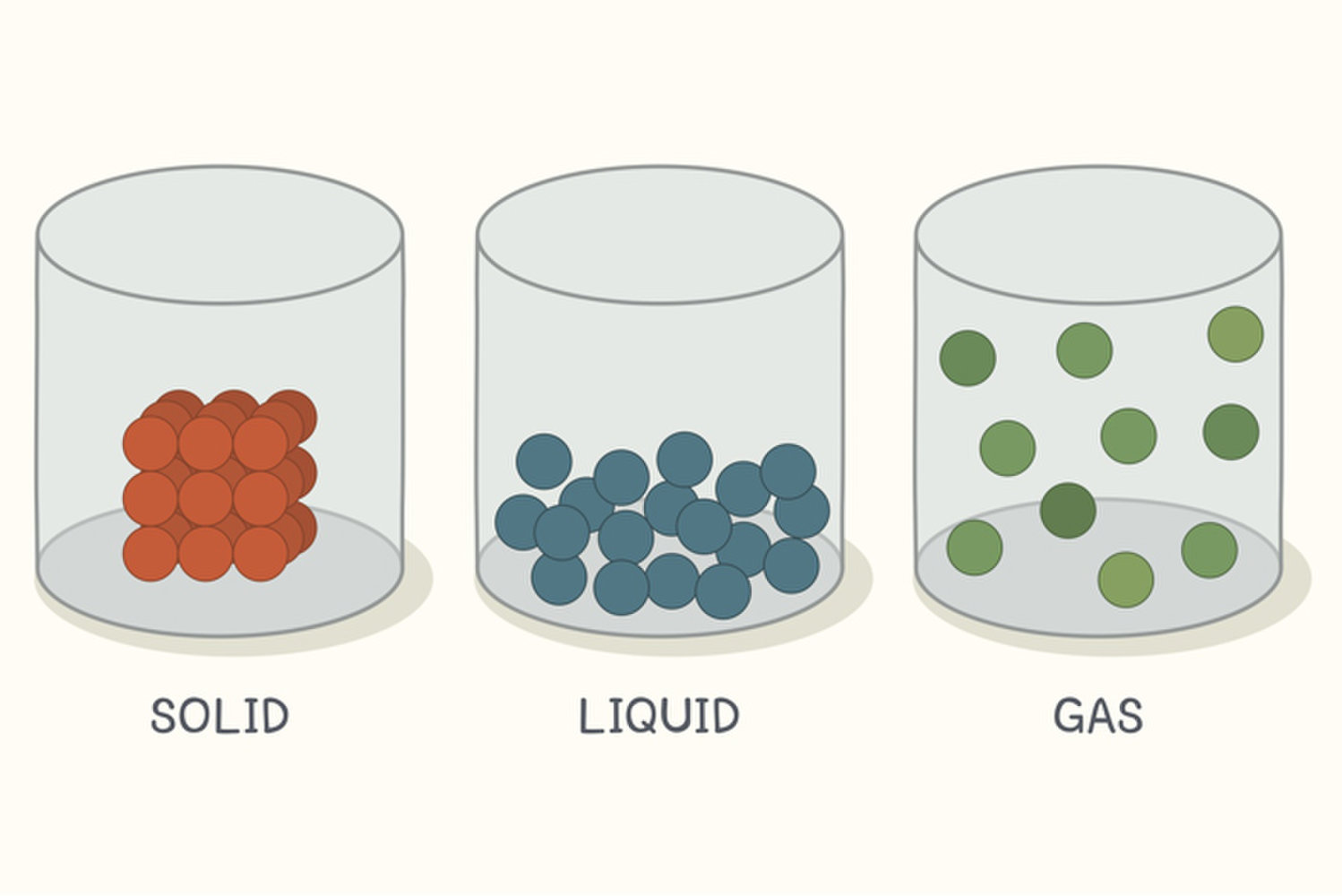
Solid Vs Liquid Density . Solids (left) are more dense than liquids: Consequently, liquids are much denser than gases. They have more atoms packed. For liquids, it is greater. Solids have a high density. Liquids typically have a lower density than. Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. The molecules of a liquid are packed relatively close together. The density of a liquid is typically about the same as the density. In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. For most solids, this expansion is relatively small, but it is far from negligible;
from socratic.org
The density of a liquid is typically about the same as the density. The molecules of a liquid are packed relatively close together. Liquids typically have a lower density than. They have more atoms packed. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. For most solids, this expansion is relatively small, but it is far from negligible; For liquids, it is greater. In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. Solids have a high density.
What are examples of gases, liquids, and solids? Socratic
Solid Vs Liquid Density Solids have a high density. Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. For most solids, this expansion is relatively small, but it is far from negligible; In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. The molecules of a liquid are packed relatively close together. The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. They have more atoms packed. For liquids, it is greater. Consequently, liquids are much denser than gases. Liquids typically have a lower density than. Solids have a high density. The density of a liquid is typically about the same as the density. Solids (left) are more dense than liquids:
From ar.inspiredpencil.com
Density Of Liquids Chart Solid Vs Liquid Density The molecules of a liquid are packed relatively close together. Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. Consequently, liquids are much denser than gases. For most solids, this expansion is relatively small, but it is far from negligible; In liquids, the particles can move around more freely, so. Solid Vs Liquid Density.
From husbynv.weebly.com
Densitet Naturvetenskap och teknik Solid Vs Liquid Density Solids (left) are more dense than liquids: Consequently, liquids are much denser than gases. In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. For most solids, this expansion is relatively small, but it is far from negligible; The difference between the densities of solids, liquids and gases is due. Solid Vs Liquid Density.
From www.dreamstime.com
Density of Matter with Gas, Liquid and Solid Particle States Outline Solid Vs Liquid Density Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. Solids (left) are more dense than liquids: The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. Solids have a high density. The difference between the. Solid Vs Liquid Density.
From chem.libretexts.org
2 The Density of Liquids and Solids (Experiment) Chemistry LibreTexts Solid Vs Liquid Density For liquids, it is greater. The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. Consequently, liquids are much denser than gases. In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. Liquids typically have a lower. Solid Vs Liquid Density.
From schoolbag.info
For a given substance, do you expect the density of the substance in Solid Vs Liquid Density Consequently, liquids are much denser than gases. For most solids, this expansion is relatively small, but it is far from negligible; In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. The molecules of a liquid are packed relatively close together. Solids (left) are more dense than liquids: The difference. Solid Vs Liquid Density.
From www.slideserve.com
PPT Density PowerPoint Presentation, free download ID4234317 Solid Vs Liquid Density Solids have a high density. They have more atoms packed. The density of a liquid is typically about the same as the density. In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c. Solid Vs Liquid Density.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid Vs Liquid Density In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. Consequently, liquids are much denser than gases. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. They have more atoms packed. The molecules of. Solid Vs Liquid Density.
From ar.inspiredpencil.com
Density Of Solids In Water Solid Vs Liquid Density Solids have a high density. For most solids, this expansion is relatively small, but it is far from negligible; The density of a liquid is typically about the same as the density. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. The molecules of. Solid Vs Liquid Density.
From www.goodsitesforkids.org
liquids of different density in layers in a tube with solid objets of Solid Vs Liquid Density The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. Consequently, liquids are much denser than gases. For most solids, this expansion is relatively small, but it is far from negligible; The difference between the densities of solids, liquids and gases is due to the. Solid Vs Liquid Density.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid Vs Liquid Density For most solids, this expansion is relatively small, but it is far from negligible; In liquids, the particles can move around more freely, so they slide over each other with some gaps between them. The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. The density of. Solid Vs Liquid Density.
From www.teachoo.com
The mass per unit volume of a substance is called density. Arrange in Solid Vs Liquid Density The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. Liquids typically have a lower density than. They have more atoms packed. Solids (left) are more dense than liquids: Consequently, liquids are much denser than gases. The molecules of a liquid are packed relatively close together. Solids. Solid Vs Liquid Density.
From www.priyamstudycentre.com
Density Formula, Definition, Measurement, Calculation Solid Vs Liquid Density They have more atoms packed. For most solids, this expansion is relatively small, but it is far from negligible; The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each. Solid Vs Liquid Density.
From sciencenotes.org
Table of Density of Common Materials Solid Vs Liquid Density Liquids typically have a lower density than. Consequently, liquids are much denser than gases. Solids have a high density. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. For most solids, this expansion is relatively small, but it is far from negligible; The difference. Solid Vs Liquid Density.
From quizzmediakathy.z21.web.core.windows.net
Density Experiment With Different Liquids Solid Vs Liquid Density Solids (left) are more dense than liquids: Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. For most solids, this expansion is relatively small, but it is far from negligible; The molecules of a liquid are packed relatively close together. The densities of the solids and liquids displayed are given. Solid Vs Liquid Density.
From www.nagwa.com
Question Video Identifying the Relative Density of a Solid and Two Solid Vs Liquid Density Liquids typically have a lower density than. For liquids, it is greater. The molecules of a liquid are packed relatively close together. The density of a liquid is typically about the same as the density. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend.. Solid Vs Liquid Density.
From www.dreamstime.com
Density and States of Matter Stock Vector Illustration of science Solid Vs Liquid Density Liquids typically have a lower density than. They have more atoms packed. The densities of the solids and liquids displayed are given for the standard temperature of 0.0 °c and the densities of solids and liquids depend. Consequently, liquids are much denser than gases. For most solids, this expansion is relatively small, but it is far from negligible; Solids, liquids,. Solid Vs Liquid Density.
From sayngon.com
Are Most Liquids Denser Than Solids? Exploring Density Differences Solid Vs Liquid Density Solids (left) are more dense than liquids: Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case. They have more atoms packed. For most solids, this expansion is relatively small, but it is far from negligible; Liquids typically have a lower density than. For liquids, it is greater. The density of. Solid Vs Liquid Density.
From byjus.com
Which of the following shows Highest interpartical spaces Highest Solid Vs Liquid Density The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. They have more atoms packed. The molecules of a liquid are packed relatively close together. The density of a liquid is typically about the same as the density. Liquids typically have a lower density than. The densities. Solid Vs Liquid Density.